What is Botulax?
FDA Approved
Available in 63+ countries (including USA, Europe & China)
#1 toxin in South Korea for 8 consecutive years

Botulax® contains the active ingredient, letibotulinumtoxinA (~900 kDa), derived from the Clostridium Botulinum CBFC26 strain
Powered by HUGEL's purification technology, Botulax® undergoes multiple purification processes to ensure its safety, potency, and high-quality standards, providing effective and optimal results for your patients.
Manufactured at an EU GMP-certified facility with:
- State-of-the-art facility that uses automated guided vehicle (AGV)
- Patented purification technology
- Strict quality control that inspects every vial
Watch Hugel Botulinum Toxin Manufacturing
Key Benefits
• Effectiveness and safety in mature patients
• High patient satisfaction rate
• Consistent potency as it goes through stricter quality control than required by global standards
• No history of antibody formation in clinical trials and pharmacovigilance
• Effective for men, who frequently present more difficult cases

Indications
It’s important to note that the specific areas treated with Botulax may vary depending on individual needs and the recommendations of the healthcare professional administering the treatment.
- 1 .
Glabellar lines
- 2 .
Lateral canthal lines
- 3 .
Blepharospasm
- 4 .
Post-stroke upper limb spasticity
- 5 .
Dynamic equinus foot deformity in children with cerebral palsy

Efficacy
BOTULAX DELIVERS GOLD STANDARD RESULTS:
- 1
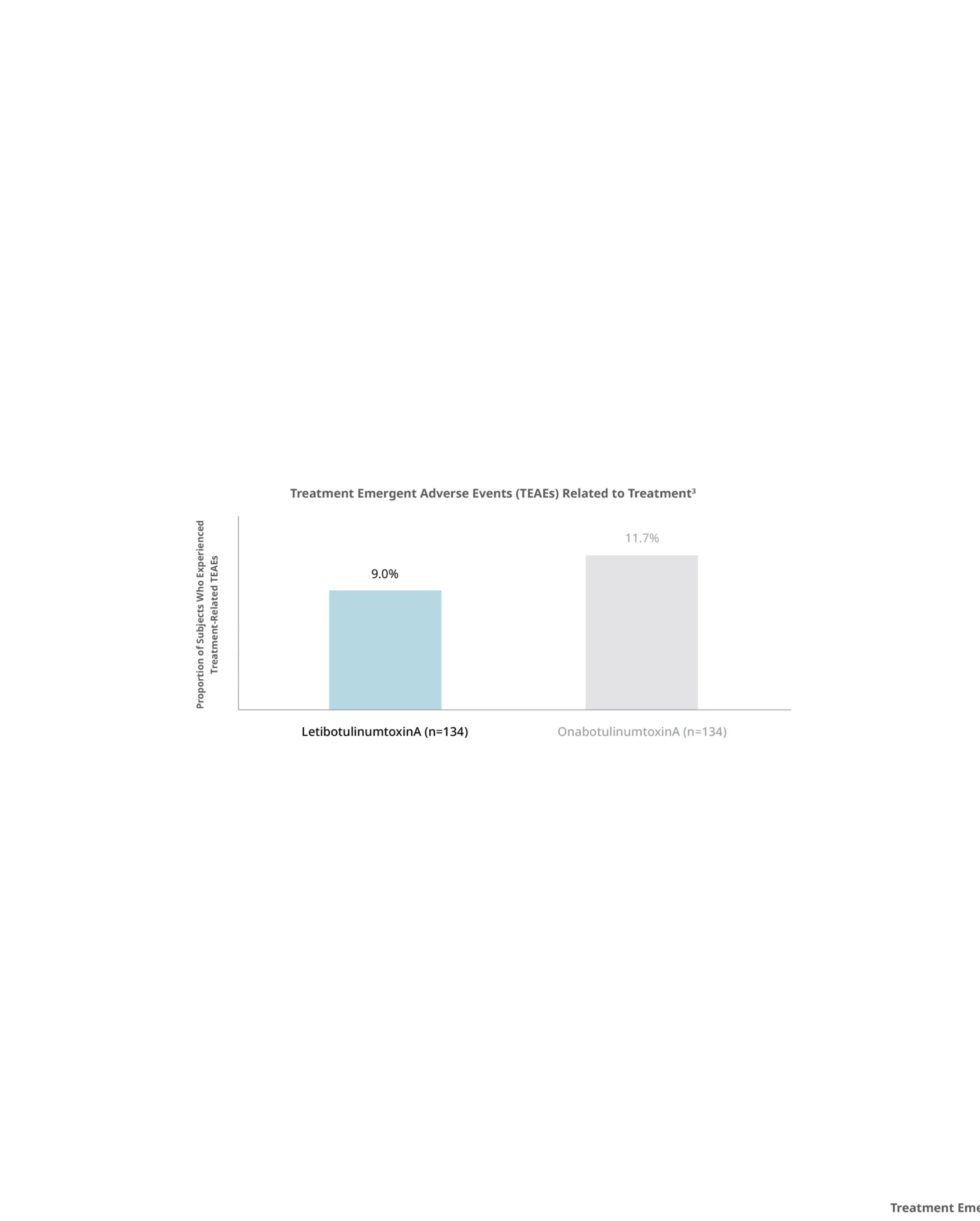
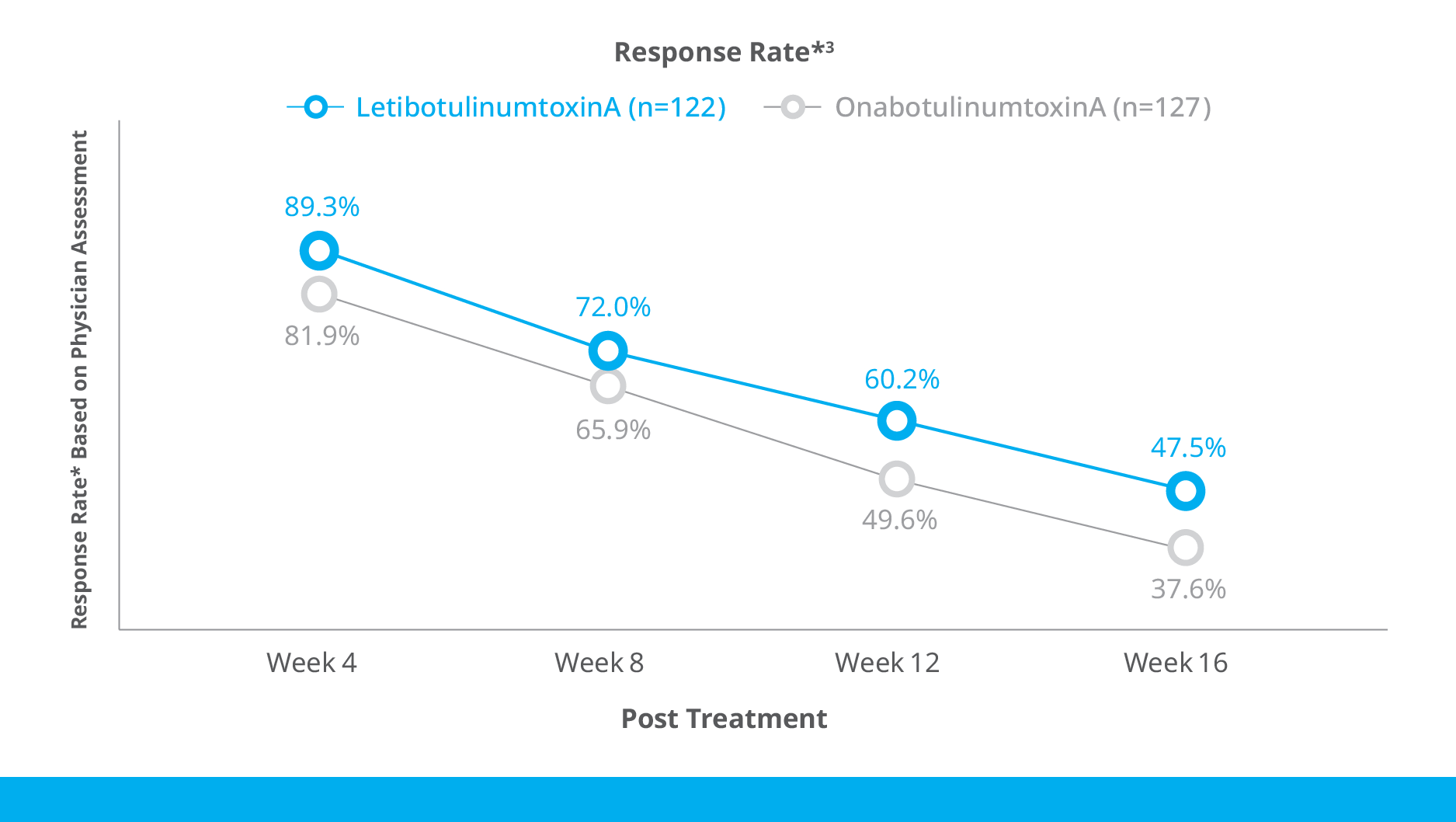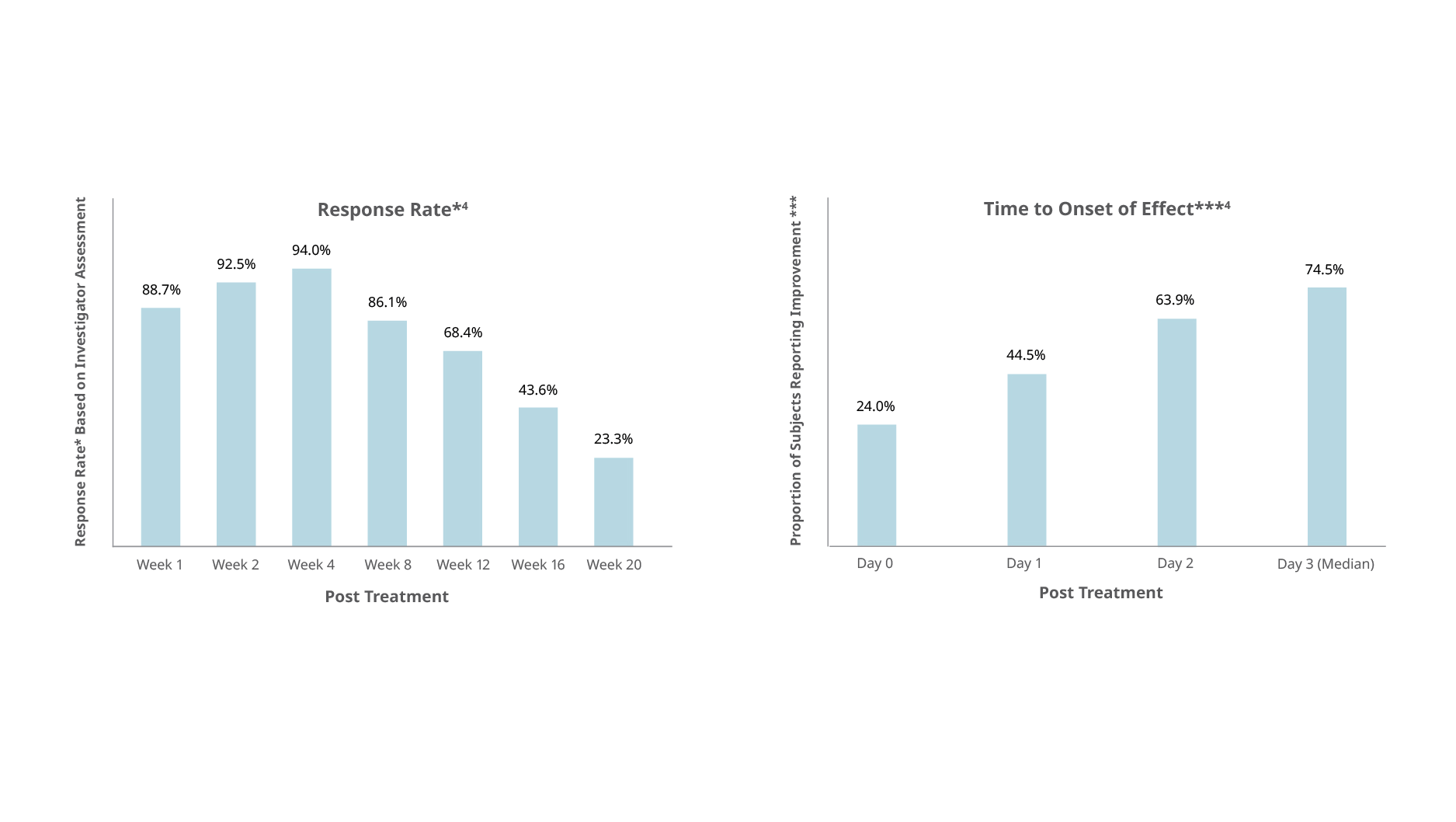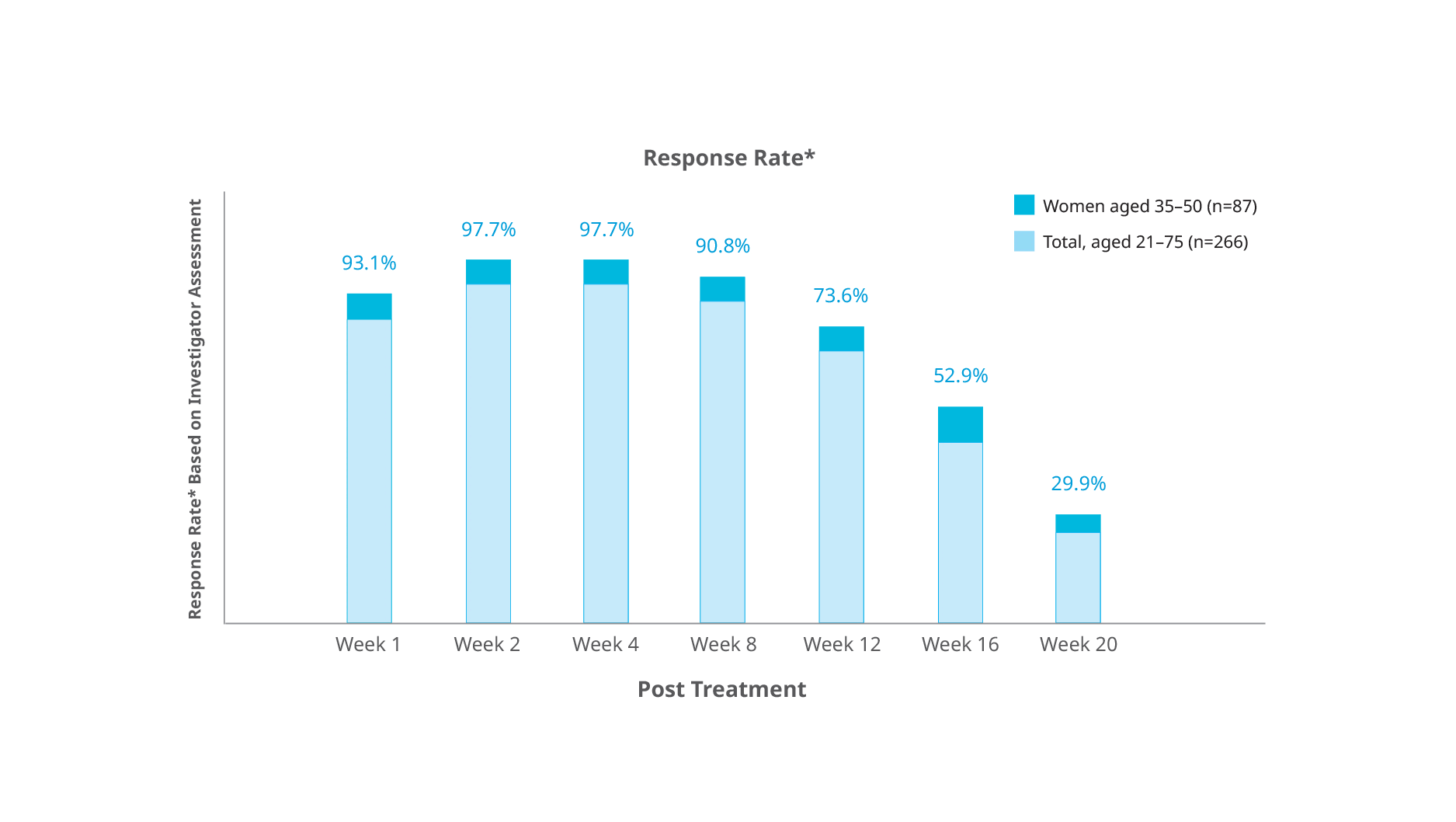
Botulax®’s efficacy in the treatment of moderate to severe glabellar lines was confirmed in multiple non-inferiority studies with over 900 subjects in total reporting a numerically higher response rates in the Botulax® group comparing with OnabotulinumtoxinA Group
Kim BJ, Kwon HH, Park SY, et al. J Eur Acad Dermatol Venereol. 2014 Dec;28(12):1761-7. - 2
Botulax®’s efficacy in the treatment of glabellar lines was further highlighted in a randomized, placebo-controlled study conducted in Europe and the U.S with below results:
- 94% response rate four weeks after treatment
- 48.5% of subjects with severe glabellar lines before treatment showing no lines at week 4
- 20 weeks duration of treatment effect
Mueller DS, Prinz V, Adelglass J, et al. Aesthet Surg J. 2022 May;42(6):677-688. - 3
Botulax® showed stronger performance in patient group of women between 35 and 50 years of age with below results:
- 97.7% response rate four weeks after treatment
- Mean duration of response of more than 19 weeks
- Median time to onset of effect was two days, with almost one third of subjects reporting improvement within 24 hours
Gold M, Taylor S, Mueller DS, et al. Aesthet Surg J Open Forum. 2024;6:ojae010. Published 2024 Feb 23. - 4
Nonetheless, Botulax® was shown to be effective in men as well, having greater facial muscle mass than women:
- 75.7% response rate at week 4, with more than half of the male subjects showing mild or no lines, as assessed by the investigator
- 22.2% experienced a 1-point reduction only one day after treatment
Safety
Botulax®’s gold standard safety in the treatment of moderate to severe glabellar lines was confirmed in three randomized, placebo-controlled studies recently conducted in Europe and the U.S. with over 1,200 subjects in total.
- No serious adverse events were reported.
- No neutralizing antibodies found in any subject after treatment.
- Consistent safety profile maintained with repeated injections.
Kim BJ, Kwon HH, Park SY, et al. J Eur Acad Dermatol Venereol. 2014 Dec;28(12):1761-7.